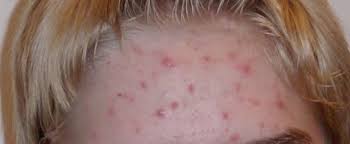
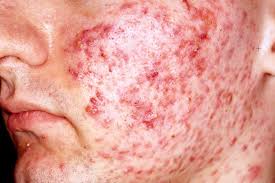
This will be a double-blind, randomized, parallel-group, vehicle-controlled, multicenter study in male and female subjects with acne vulgaris. Subjects will be admitted into the study only after written informed consent has been obtained and all of the inclusion and none of the exclusion criteria have been met.
Randomized subjects will apply the Investigational Product (IP) to their entire face once daily at bedtime for approximately 84 days (12 weeks). Follow-up assessments of safety and efficacy will be done about every 28 days through Day 84. Safety will also be monitored by telephone contact at Day 7. |